HOME
Chemists teach neural networks to predict properties of compounds
Graphical abstract.
A new joint Russian-French-Japanese team has developed a computational model able to predict the properties of new molecules based on the analysis of fundamental chemical laws. The study, titled "Using AI methods for the planning of chemical synthesis," is published in the Journal of Chemical Information and Modeling.
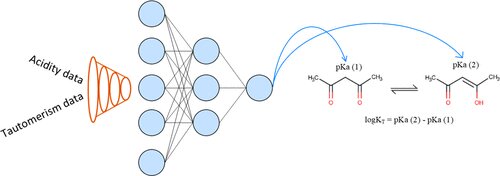
Associate Professor Timur Madzhidov says, "We offered a way to insert the preexisting chemical equations into some frameworks of machine learning. It was tested on the predictions of tautomeric constants and acidity, which are linked by the Kabachnik equation. Using the functional interdependency between them, the neural network learns how to predict both these properties."
Prototropic tautomerism is the phenomenon of reversible isomerism, in which isomers (substances having the same qualitative and quantitative composition, but differing in structure and properties) easily transition into each other due to the transfer of a hydrogen atom.
"Tautomeric transformations are very common for organic compounds, being known for about half of all discovered compounds. For example, one of the mechanisms of spontaneous mutations is tied to the tautomeric transformations of DNA nucleic base. That why tautomerism must be taken into account when registering new compounds, during the computer design of new medications, and the search for molecules with preconditioned properties," says Madzhidov.
The results of this research could increase the precision of prediction of physicochemical properties of designed medication and materials, as well as correctly forecast the parameters of chemical reactions.
News Source