HOME
Scientists probe the chemistry of a single battery electrode particle both inside and out
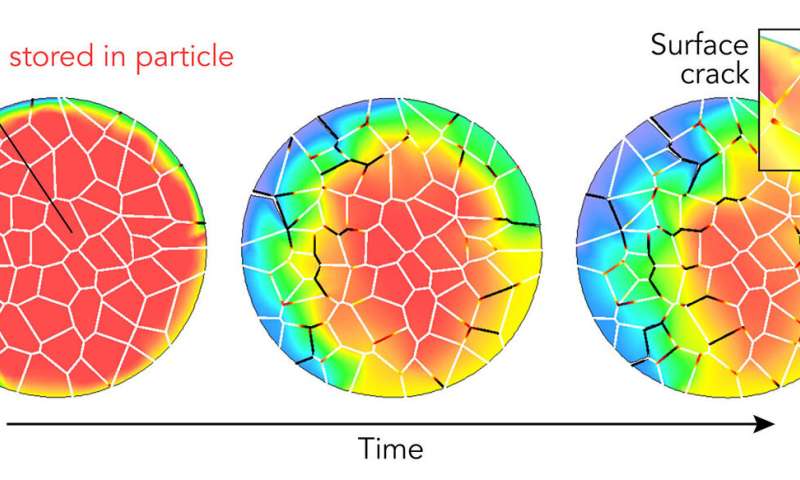
A simulation based on X-ray experiments at SLAC shows what happens to a single battery electrode particle when it is charged over the course of 12 minutes. The particle swells and shrinks as lithium ions enter and leave, causing the particle to crack (black lines). Then electrolyte seeps into those cracks and damages the interior, reducing the volume where lithium ions can be stored (reddish area) and thus the particle's ability to store energy. The study found that interactions between the particle's surface and interior are important for understanding these damage patterns. Credit: S. Li et al., Nature Communications, 2020
The particles that make up lithium-ion battery electrodes are microscopic but mighty: They determine how much charge the battery can store, how fast it charges and discharges and how it holds up over time—all crucial for high performance in an electric vehicle or electronic device.
Cracks and chemical reactions on a particle's surface can degrade performance, and the whole particle's ability to absorb and release lithium ions also changes over time. Scientists have studied both, but until now they had never looked at both the surface and the interior of an individual particle to see how what happens in one affects the other.
In a new study, a research team led by Yijin Liu at the Department of Energy's SLAC National Accelerator Laboratory did that. They stuck a single battery cathode particle, about the size of a red blood cell, on a needle tip and probed its surface and interior in 3-D with two X-ray instruments. They discovered that cracking and chemical changes on the particle's surface varied a lot from place to place and corresponded with areas of microscopic cracking deep inside the particle that sapped its capacity for storing energy.
News Source